Colosafe First NMPA-approved stool DNA test kit for early detection of colorectal cancer
02Nov
Colosafe is the stool DNA test kit for early detection of colorectal cancer. It was the first approved of its kind by NMPA on November 20, 2018. This test can accurately detect and interpret aberrant DNA messages (human SDC2 methylation) from stool samples, enabling physicians to identify cancerous lesions early to prevent the development of colorectal cancer (CRC) at its initial stages. Consequently, the prevention and potential cure of CRC can be achieved. Colosafe has been implemented in over 700 healthcare institutions in China and has gained significant clinical recognition from professionals and experts.

Biological Principles
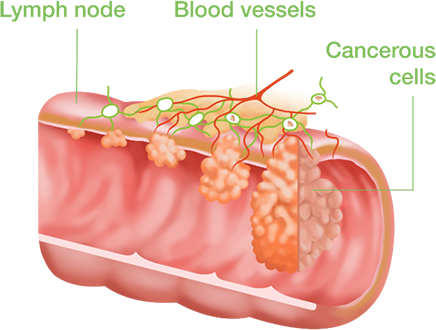
- Epithelial cells, including tumor cells, exfol
- Detecting mutated DNA from cancer cells
- Collection and Detection of Human DNA
- Achieve early detection of colorectal cancer (CRC)
- Detecting mutated DNA from cancer cells
How to Use Colosafe Stool Collection Device
Comments



